Your views

Your feedback is vital to us as we continue to increase the quality of our services.
You are here:
Date: 27 January 2025
Time: 17:03
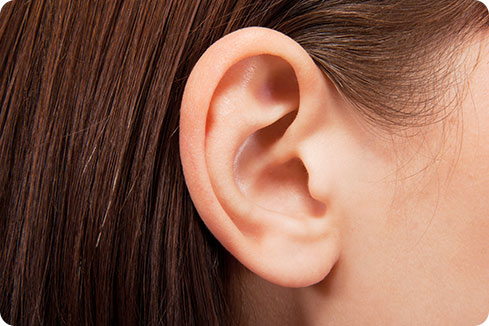
QUIET-1 tinnitus study
Story posted/last updated: 02 April 2015
Tinnitus (ringing, hissing or buzzing sounds in your ears or head) affects one in 10 adults in the UK. QEHB are pioneering the development of a new drug to treat tinnitus through the QUIET-1 study.
The QUIET-1 study is a clinical study to determine whether or not a new test drug, AUT00063, can reduce symptoms of tinnitus in comparison to a placebo after four weeks of treatment.
If you are 18 years or over and have had continual tinnitus for more than 6 months but less than 18 months, but are otherwise healthy, you may be eligible to take part. Screening will be required to make sure that you meet certain eligibility criteria and the final decision on study enrolment will be taken by the trial doctor.
AUT00063 has completed initial “Phase I” safety studies in healthy volunteers. No adverse effects of the drug on measures of hearing were observed in any of the subjects. AUT00063 is an orally active preparation that will be taken as 4 capsules once daily with food.
Participants will be reimbursed for giving up their time and any reasonable travel expenses.
If you are interested in taking part in the study or want more information, please contact the ENT Research Office.
Tel: 0121 414 9276 / 0121 414 8754

Getting here
Information about travelling to, staying at and getting around the hospital.

Jobs at UHB
A great place to work. Learn why.
news@UHB


RSS feed
Subscribe to our news feed


